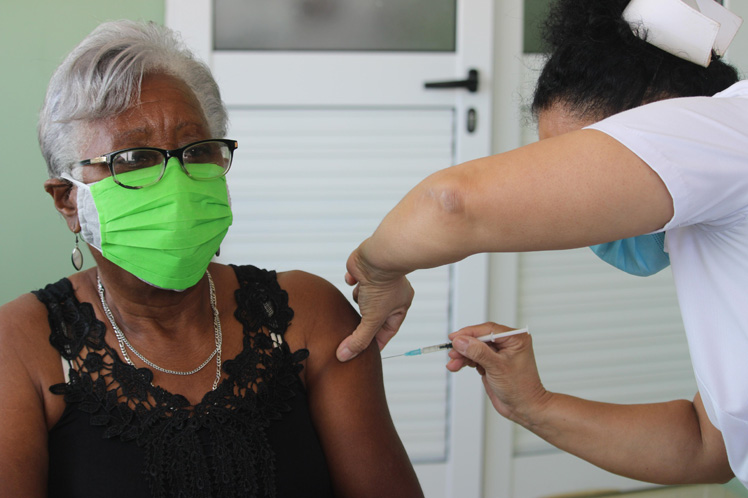
This is phase II A of clinical trials of this vaccine candidate from the Finlay Vaccines Institute (IFV), whose study is being carried out at the Hematology and Immunology Institute, in this capital.
The vaccination of a score of people of the 450 convalescing patients included in this phase of the study started on Saturday.
Dr. Arturo Chang, main researcher of the vaccine, told Agencia Cubana de Noticias that people between 60 and 80 years of age, who had suffered from Covid-19 without serious adverse effects, were included in the current phase.
When the first results of the analyses are available, phase II B will begin, which involves the immunization of another 430 volunteers (convalescents between 19 and 80 years of age), he said.
This drug, together with other vaccines, reinforces people’s immune response, so it is currently being administered to convalescing individuals and as part of intervention studies with Soberana 02, which also includes a third shot of Soberana Plus in its vaccination schedule.
Dr. Rolando Ochoa, researcher and full professor at IFV, has explained that the preliminary results of the previous phase show that the vaccine is safe; no severe adverse effects are reported, and the people who took part in it had a high immune response.
Cuba has another four Covid-19 vaccine candidates: Soberana 01 and Soberana 02, from IFV; and Abdala and Mambisa, from the Center of Genetic Engineering and Biotecnology.
rly/aph/mem/rbp